Transcranial magnetic stimulation (TMS) is a noninvasive medical treatment that uses magnetic fields to stimulate specific areas of the brain, primarily for depression and other psychiatric conditions. First developed in 1985, this FDA-approved therapy has evolved into a widely used option for patients who haven’t responded well to traditional treatments like medication or psychotherapy.
TMS works by delivering targeted magnetic pulses to nerve cells in the brain while patients remain awake and alert in a doctor’s office, with sessions requiring no surgery, sedation, or recovery time. The procedure allows individuals to drive themselves home immediately after treatment and continue their daily activities without interruption.
Understanding how TMS therapy works, what happens during treatment sessions, and what results patients can expect helps individuals make informed decisions about whether this option fits their needs. This guide examines the scientific mechanisms behind TMS, the treatment experience from consultation through follow-up care, and the safety profile and outcomes documented in clinical practice.
TMS Therapy: Process, Mechanisms, and Session Experience
Transcranial magnetic stimulation delivers targeted magnetic pulses to specific brain regions to modulate neural activity, primarily targeting areas like the dorsolateral prefrontal cortex. The therapy involves precise stimulation parameters and specialized electromagnetic coils that create non-invasive brain stimulation through carefully controlled sessions.
Overview of Transcranial Magnetic Stimulation
Transcranial magnetic stimulation is a non-invasive brain stimulation technique that uses electromagnetic coils to generate magnetic pulses. These pulses pass through the skull to reach specific brain regions without requiring surgery or anesthesia.
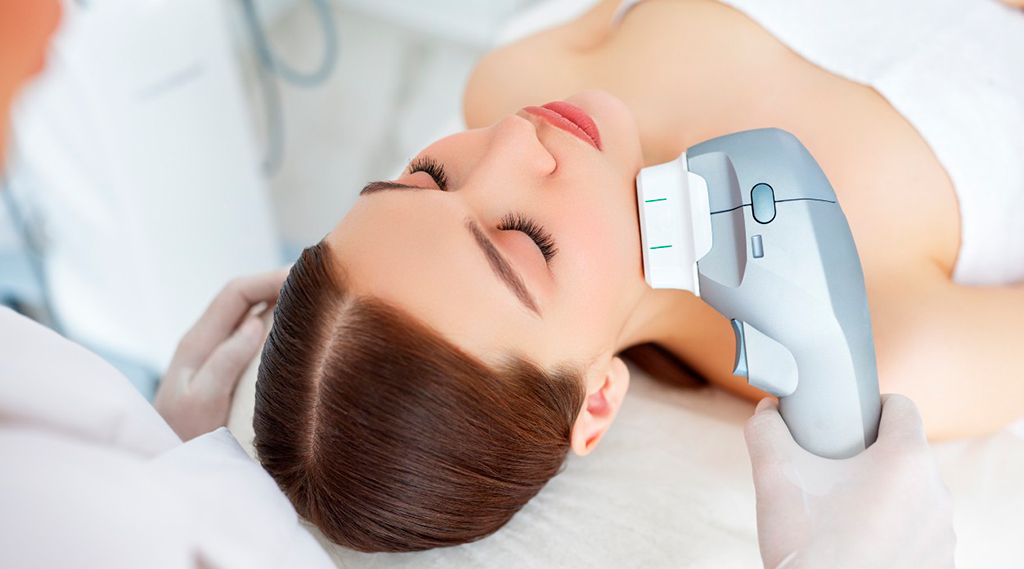
The procedure takes place in a clinical setting with the patient fully awake and alert. A trained technician positions a TMS coil against the patient’s scalp, typically over the left prefrontal cortex or dorsolateral prefrontal cortex. The magnetic field generated penetrates approximately 2-3 centimeters into brain tissue.
Each session lasts between 20 to 40 minutes depending on the protocol used. Patients remain seated comfortably in a treatment chair throughout the procedure. They can return to normal activities immediately after each session without recovery time.
How TMS Works in the Brain
The electromagnetic coil generates rapidly changing magnetic fields that induce small electrical currents in targeted brain regions. These currents activate neurons and trigger action potentials, leading to neuromodulation of neural circuits involved in mood regulation.
Repetitive transcranial magnetic stimulation causes lasting changes in synaptic plasticity and neurotransmitter function. The therapy increases blood flow to underactive brain areas, which can be measured using PET imaging. These physiological changes help rebalance neural pathways that have become dysregulated in depression.
The magnetic pulses specifically target the dorsolateral prefrontal cortex, a region consistently shown to have reduced activity in depression. Stimulation of this area enhances connectivity with deeper limbic structures. Some protocols also target the temporoparietal cortex for specific conditions.
Step-by-Step TMS Session Process
The initial consultation includes a thorough psychiatric evaluation and brain mapping to identify optimal coil placement. The treatment team measures the patient’s motor threshold by stimulating the motor cortex until a thumb twitch occurs. This determines the appropriate stimulation intensity for therapeutic sessions.
During each TMS session, the patient sits in a reclined chair while the technician positions the coil. The pulse generator delivers magnetic pulses in a specific pattern based on the prescribed protocol. Patients typically hear clicking sounds and feel tapping sensations on the scalp during stimulation.
Common session components include:
- Precise coil positioning using anatomical landmarks
- Administration of magnetic pulses in trains or bursts
- Brief rest periods between stimulation trains
- Monitoring for comfort and side effects
- Documentation of treatment parameters
Standard treatment courses involve daily sessions five days per week for 4-6 weeks.
Types of TMS: rTMS, Deep TMS, and Theta Burst Stimulation
Repetitive TMS (rTMS) delivers magnetic pulses in regular, repeated patterns using a figure-8 coil. High-frequency rTMS (typically 10 Hz) produces excitatory effects, while low-frequency protocols (1 Hz) create inhibitory effects. Treatment sessions last 30-40 minutes.
Deep TMS uses an H-coil design that reaches deeper brain structures up to 4-5 centimeters below the skull surface. This technology stimulates broader brain regions compared to standard figure-8 coils. Deep transcranial magnetic stimulation may access limbic structures more directly.
Theta burst stimulation (TBS) delivers pulses in specific burst patterns that mimic natural brain rhythms. Intermittent theta burst stimulation (iTBS) provides treatment in just 3-10 minutes, significantly shorter than conventional protocols. TBS uses different stimulation parameters with bursts of three pulses delivered at 50 Hz, repeated at 5 Hz intervals.
|
TMS Type |
Coil Design |
Session Duration |
Penetration Depth |
|
Standard rTMS |
Figure-8 coil |
30-40 minutes |
2-3 cm |
|
Deep TMS |
H-coil |
20-30 minutes |
4-5 cm |
|
iTBS |
Figure-8 coil |
3-10 minutes |
2-3 cm |
TMS Therapy Safety and Outcomes
TMS therapy has demonstrated a favorable safety profile with specific eligibility requirements that help minimize risks and optimize treatment effectiveness. Research from systematic reviews and randomized controlled trials has established clear evidence for its efficacy in treating depression and other psychiatric disorders, with outcomes typically measured through standardized assessments like the PHQ-9.
Eligibility Criteria and Patient Selection
A comprehensive pre-TMS evaluation includes a detailed review of the patient’s medical, surgical, neurologic, and psychiatric history. Clinicians assess current and prior medications, treatment dose, duration, and outcomes regarding safety, tolerability, and efficacy.
Patients with certain conditions require careful screening. Individuals with epilepsy face restrictions due to seizure risk, while those with metallic implants near the treatment site may be excluded. The evaluation process also examines current medications that could lower seizure threshold.
Motor threshold mapping occurs during the first appointment to personalize stimulation levels. This procedure measures the appropriate magnetic pulse strength for each patient’s specific treatment needs.
TMS is particularly considered for treatment-resistant depression (TRD) when patients have not responded adequately to standard interventions. The therapy also shows potential application for obsessive-compulsive disorder (OCD), though depression remains the primary FDA-approved indication.
TMS Therapy Safety Profile and Side Effects
TMS is a non-invasive procedure that requires no surgery or cutting of the skin. The most common side effect is scalp discomfort at the treatment site during or after sessions, which typically decreases as treatment progresses.
Common Side Effects:
- Scalp discomfort or pain
- Headache
- Tingling or twitching of facial muscles
- Lightheadedness
Serious adverse events are rare. Seizure represents the most concerning risk, occurring in less than 0.1% of treatments according to safety guidelines. Current safety protocols, updated from the original 2009 guidelines, provide comprehensive frameworks for minimizing risks in both research and clinical settings.
Most patients tolerate TMS well without medication adjustments. The treatment does not require anesthesia or sedation, allowing patients to drive themselves to and from appointments and resume normal activities immediately.
Efficacy and Outcomes for Psychiatric and Neurological Disorders
TMS demonstrates established effectiveness for major depressive disorder (MDD) and treatment-resistant depression. Clinical trials show that repetitive TMS (rTMS) produces lasting effects on brain function by improving communication between brain cells in regions responsible for mood regulation.
Evidence-Based Applications:
- Depression/TRD: Strong evidence from multiple randomized controlled trials
- OCD: FDA-approved with documented efficacy
- Migraine: Emerging evidence for prevention
- PTSD: Promising results in preliminary studies
- Smoking cessation: Research ongoing
Studies measuring outcomes through standardized tools like the PHQ-9 demonstrate clinically significant improvement in depressive symptoms. The therapy’s effectiveness for psychiatric disorders has positioned it as a key option in the therapeutic landscape for patients who have not responded to traditional treatments.
Research continues to explore TMS applications for stroke rehabilitation, Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, spasticity, and bipolar disorder. While these applications show potential, the evidence base remains strongest for depression and OCD treatment.
NC
27617